Plant Biology / BIOL 3306
Table of Contents
- 1. Introduction
- 2. Plant cells
- 3. Plant Tissues 1
- 4. Plant Tissues 2
- 5. Introduction to plant identification
- 6. Herbarium visit
- 7. Roots 1
- 8. Roots 2
- 9. Shoots and primary growth
- 10. Leaves 1
- 11. Leaves 2
- 12. Flowers and secondary growth
- 13. Secondary growth 2
- 14. Plant ID workshop #2
- 15. Phylogenies and taxonomy
- 16. Origins of the plants
- 17. Bryophytes
- 18. Seedless vascular plants
- 19. Gymnosperms
- 20. Angiosperms
- 21. Angiosperm Flowers
- 22. Fruits and seeds
- 23. Photosynthesis: light reactions
- 24. Photosynthesis: Carbon fixation
- 25. Photosynthesis: C4 and CAM
- 26. Water movement 1
- 27. Water movement 2
- 28. Phloem and Introduction to plant hormones
- 29. Control of growth
- 30. Life history
- 31. Fire 1
1. Introduction
1.1. Syllabus and logistics
1.2. Why Plants?
1.3. Plant form
- Wouldn’t it be great to be able to photosynthesize?
- Animals that photosynthesize? Elysia viridis

- Notes B_note
This sea slug can retain chloroplasts of the algae it eats. There is some debate on how useful but most recent evidence suggests that the slug can obtain carbon from this source. There is also evidence of horizontal gene transfer.
Refs:
http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0120874
- Notes B_note
- Key aspects of plant form
- A herbaceous Angiosperm
Phylogenetically: This is a land plant, vascular plant, seed plant, flowering plant.

- Many solutions to every problem

- Example: dealing with drought

- Example: dealing with drought

- Example: dealing with drought

- Example: dealing with drought

1.4. A history of plants
- Oxygen in the atmosphere
- Origins of photosynthesis
- First autotrophs: at least 3.4 billion years ago
- Photosynthesis in its current form (O2 producing), evolved ≈ 2.7 billion years ago in cyanobacteria.
- What are the plants?
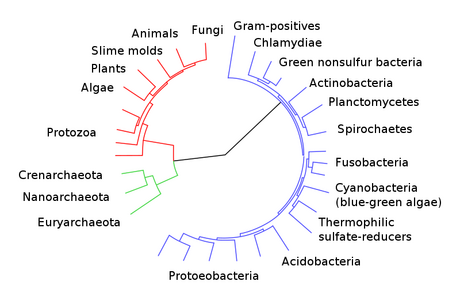
- Overview phylogeny of the plants

- Green plants and specifically, the land plants are the focus of this course
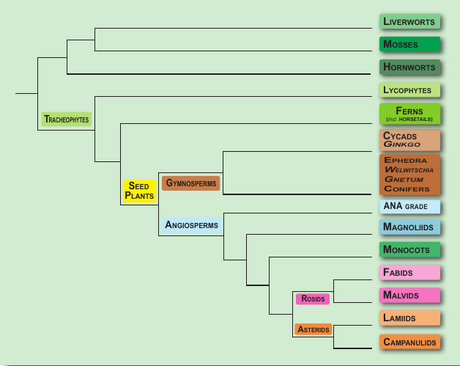
2. Plant cells
2.1. Cell overview
2.2. Internal components
- Endomembrane system
- Endomembrane system

- Organelles surrounded by one membrane
- Vacuole
Mature plant cells often have one large vacuole that is 90%+ of the cell. Why?
- Peroxisome
Important in metabolism, especially dealing with byproducts of photorespiration.
- Lecture notes on vacuoles and peroxisomes B_note
- Mature plant cells often have one large vacuole that is 90%+ of the cell. Why?
- Contains “cell sap” (mostly water). Pressure in this vacuole pushes against cell wall and keeps cell rigid and plant from wilting.
- Contains mostly water, but also solutes such as Ca2+, K, Cl-, NA+, HPO42- (cell sap)
- Can contain pigments (anthocyanins). Fall color in leaves.
- Can store toxins and secondary metabolites used in defense (eg nicotine). Toxic to plant!
- glyoxysomes (specialized peroxisomes) help convert stored fats to sucrose in seed germination. Plant and fungi specific!
- peroxisomes are self-replicating but must import materials no DNA)
- Vacuole
- Organelles surrounded by two membranes
And have their own DNA
- Plastids
Chloroplasts, leucoplasts, and chromoplasts.
- Mitochondria
- Nucleus
- Lecture notes on plastids B_note
- Chloroplasts are site of photosynthesis
- Chromoplasts lack chlorophyll (can develop from chloroplasts). Synthesize carotenoids responsible for some flower and fruit colors.
- Leucoplasts are unpigmented. Most important are starch synthesizing amyloplasts. Also plastids that store fats or proteins.
- proplastids are the undifferentiated forms (eg in seeds).
- plastids must reproduce by division.
- mitochondria - similar and often congregate where energy is needed in cell (eg at membrane involved in active transport).
- Both plastids and mitochondria have DNA that codes for some, but not all of their own polypeptides. Semi-autonomous.
- plastids can change into one another.
- Plastids
- Chloroplast

- Chloroplasts
The site of photosynthesis
- Light energy converted to short term ATP and NADPH in the thylakoid stacks (grana)
- ATP and NADPH and CO\(_2\) converted to sugar in the stroma
- Chromoplasts of a red pepper fruit
2.3. Cell wall and cytoskeleton
- Cytoskeleton
Protein filament types: actin filaments and microtubules
Active in organizing cell growth and division and cytoplasmic flow.
- Cytoplasmic streaming exmaple
- Primary cell wall
Deposited before and during cell growth.

- Cell wall components
- Cellulose (polysaccharide) bundles are interlocked with hemicellulose, pectins, and glycoproteins.
- Cutin, suberin, and waxes can be part of the cell wall
- Cellulose extrusion

- Cellulose extrusion

- Secondary cell wall

- Pits and plasmodesmata

- Pits and Plasmodesmata

3. Plant Tissues 1
3.1. Modular growth
- Key aspects of plant growth
- Unlike animals, plants have partially differentiated meristems throughout their body and throughout their life
- Plants are modular
- Plant growth is indeterminate
- Plants are sessile but can use growth as their behavior instead of motility
- Plants have three vegetative organs: stems, leaves, and roots
- Modular shoot growth

- Repeated modules

- Huge variation in size

- Huge variation in size

- Shoot apical meristem

- Root apical meristem

3.2. Tissue types
- Primary meristems and tissues
Apical meristems produce daughter cells that partially differentiate into primary meristems.
- Cell differentiation

- Distribution of major tissue types

- Ground tissue system
- Parenchyma cells
- In leaves, contain chloroplasts. In other organs, may store starch. Totipotent
- Collenchyma cells
- Typically Long and thin, support plant body. Primary cell walls of uneven thickness.
- Sclerenchyma cells
- Fibers and sclerids. Thick secondary cell wall. Usually dead at maturity.
- Parenchyma cells: leaves

- Parenchyma cells: roots

- Transfer cells (parenchyma)

- Collenchyma cells

- Scerenchyma cells: fibers


- Yucca fibers

- Yucca fibers

- Yucca fibers on leaf margin
As well as around vascular bundles
- Yucca fibers: baskets and rope

- Scerenchyma cells: sclerids

4. Plant Tissues 2
4.1. Vascular tissue system
- Distribution of major tissue types

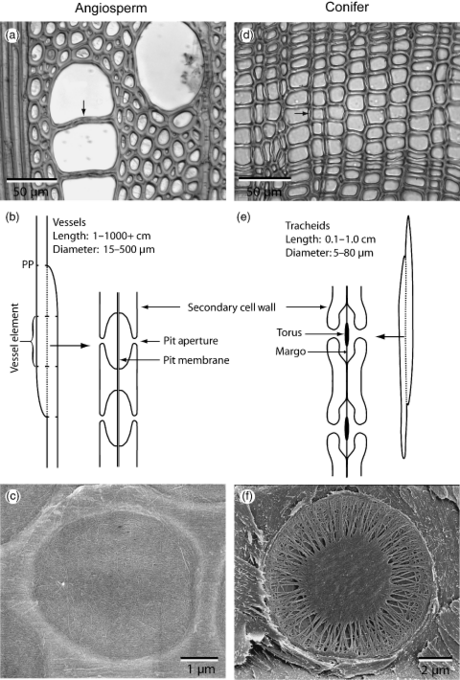
- Xylem tissue
- Tracheid and two vessel elements

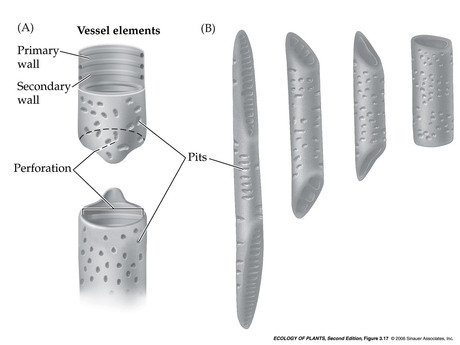
- Vessel elements

- Formation of vessel elements

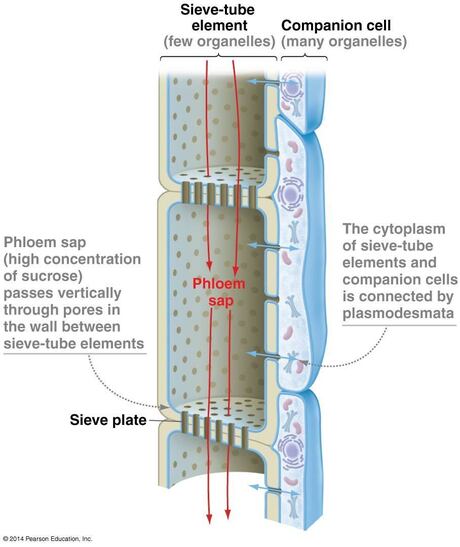
- Phloem tissue
- Sieve elements (alive with protoplast):
- sieve elements
- General term and term for simplest type found in non seed plants
- sieve cells
- with associated albuminous cells (found in gymnosperms)
- sieve tube elements
- with associated companion cells (found in angiosperms)
- Other cell types in phloem:
parenchyma, fibers, sclerids
- Sieve elements (alive with protoplast):
- Sieve tube element and companion cell

- Sieve tube element and companion cell in barley

- Sieve tube elements in squash

- Sieve tube elements in squash

5. Introduction to plant identification
6. Herbarium visit
- Meet in 102, then go to herbarium.
7. Roots 1
7.1. Root Overview
- Taproots and fibrous roots

- Lecture notes B_note
Two plants that you can find growing wild around Lubbock in the shortgrass prairie. Liatris punctata and Aristida purpurea
In monocots, the original primary root is short lived and the root system mostly develops from adventitious roots – roots that develop from the stem. This is the same thing as when a shrub with a drooping branch forms roots where the branch touches the ground.
Most of the root length and area is in the fine roots / feeder roots.
Some roots have been measured very deep (53 m in mesquite in arizona in a mine), 68 meeters for a Kalahari shrub.
- Lecture notes B_note
- Root external overview

- Review: Distribution of major tissue types

- Root growth

- Lecture notes B_note
- regions: cell division, cell elongation, maturation
- Root hairs develop behind region of elongation, older parts of roots lose hairs (die)
- phloem develops early – growing tip needs carbon source, xyelm mautres behind this
- Endodermis and casparian strip develops as maturation occurs
- Lecture notes B_note
- Root cap and apical meristem

- Root cap - sensing gravity
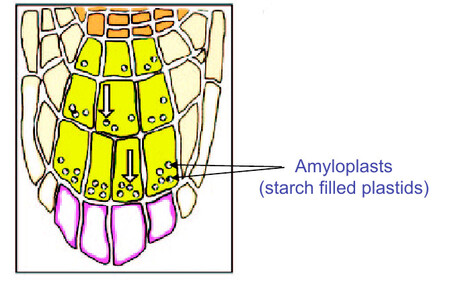
- Growth without the root cap
- Root cap and border cells

- Root hairs are single cells

- Root hairs
8. Roots 2
8.1. Primary growth structure continued
- Rootcap, colummella, and quiescent center
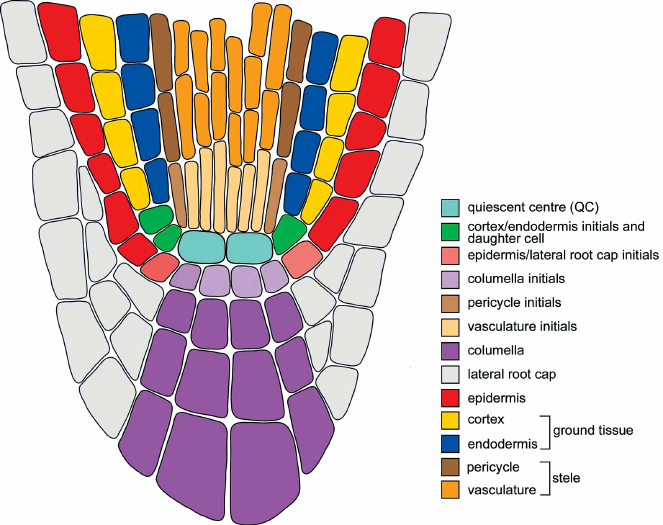
- Exodermis

- Lecture notes B_note
In most angiosperms, another layer of cells from casparian strips: the exodermis. Can prevent water loss. This can be continuous and fully suberized in plants form arid environments, or can be patchy and discontinuous. Passage cells are thin cells in exodermis that allow cross mebrane transport (especially Ca and Mg).
More on Casparian strip. It is not just suberin. But other waxes as well as lignin as in secondary cell walls.
- Lecture notes B_note
- Vascular arrangement: eudicots

- Vascular arrangement: monocots

- Endogenous branching
- Endogeneous branching

8.2. Secondary growth
9. Shoots and primary growth
9.1. Primary growth
9.2. Vascular tissue arrangement
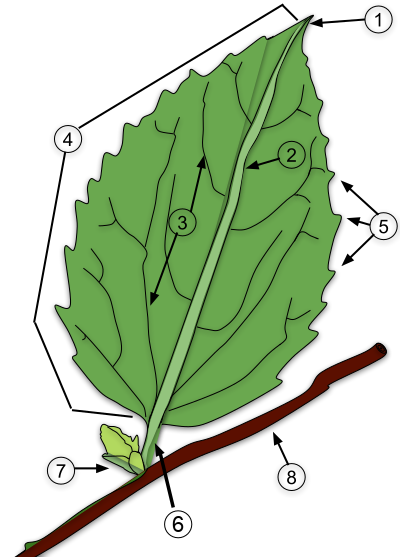
10. Leaves 1
10.1. Vascular connections
10.2. Leaf nomenclature and variation
10.3. Leaf modifications
- Short-shoot long-shoot architecture
Example: Fouqueria splendens (Ocotillo)

- Long shoot growth

- Petiolar spines forming from leaf midrib

- Short shoots

- Tendrils: Pea (Fabaceae)

- Cactus spines (Cactaceae)

- Cactus spines (Cactaceae)

- Leaves on Opuntia (Cactaceae)

- Leaves on Opuntia (Cactaceae)

- Plant sharp parts
- Spines
- Modified leaves or part of leaf (eg modified stipules in Acacia and Prosopis)
- Thorns
- Modified stems
- Prickles
- Outgrowths of the cortex and epidermis
- Thorns on Ziziphus obtusifolia (Rhamnaceae)

- Prickles on Rosa

- Stipular spines ion Prosopis glandulosa (Fabaceae)

- Leaf variation: traps

- Bulb: Allium cepa (Amaryllidaceae)

11. Leaves 2
11.1. Stem modifications
11.2. Leaf morphology
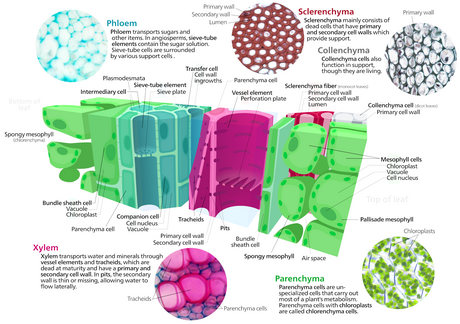
- Leaf anatomy overview
- Mesophyll
- specialized for photosynthesis. Palisade parenchyma and spongy parenchyma
- Epidermis
- Stomata, cuticle, trichomes
- Vascular bundles
- Smaller veins have bundle sheath cells, larger may be embedded in more sclerified tissue.
- Leaf anatomy
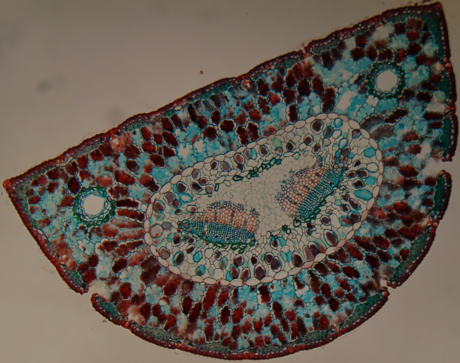
Kelvin Ma 2014.
- Transverse cross section Syringia vulgaris (lilac, Oleaceae)

- Syringia vulgaris

- Transverse cross section Syringia vulgaris

- Paradermal section Syringia vulgaris

- Lower epidermis Syringia vulgaris

- Variation: a xeriphyte
Nerium oleander (Apocynaceae)

- Variation: xeriphyte
Nerium oleander (Apocynaceae)

- Variation: Nymphaea odorata (Nymphaceae)

- Variation: Nymphaea odorata (Nymphaceae)

- Maize stomata with subsidiary cells
Stomata in grasses occur in rows

- Grass leaves: sugercane
Kranz anatomy

- C3 grass: Poa annua

- C3 grass: Poa annua

- Pine needles (Pinus sylvestris, Pinaceae)
12. Flowers and secondary growth
12.1. Flower development
- Flower development from apical meristem
Neptunia pubescens (Fabaceae)

- Flower development from apical meristem
Neptunia pubescens

- Flower development from apical meristem
Neptunia pubescens

- Flower development from apical meristem
Neptunia pubescens

- 5 stamen primordia
- Flower development from apical meristem
Neptunia pubescens

- Flower development from apical meristem
Neptunia pubescens

- Neptunia pubescens

Photo by Bob Peterson
- Neptunia pubescens

12.2. Secondary growth
- Seasonal growth cycles
- Puya raimondii (Bromeliaceae)

By Pepe Roque - Own work, CC BY-SA 3.0,
- Deciduous vs evergreen perennials
- Deciduous
- lose leaves during cold season (winter-deciduous) or dry season (drought-deciduous)
- Evergreen
- Have some leaves all year. But leaf lifespan can be very short (a month) or long (multiple years)
- Secondary growth
- increases girth of plant body
- occurs after primary growth and usually in second year/season of growth
- occurs from two lateral meristems: The vascular cambium and the cork cambium
- Vascular cambium of stem

13. Secondary growth 2
13.1. Vascular cambium
13.2. Periderm
- The cork cambium
Makes the periderm composed of three parts
- The cork cambium itself
- Forms cork to the outside - suberin and wax layers, dead at maturity)
- Forms phelloderm to the inside (resembles cortical parenchyma from primary growth)
- Tilia americana year one

- Tilia americana year two

- Tilia americana year three

- Lenticel in Aristilochea spp (Aristolochioideae)

- Bark, heartwood, sapwood

- Bark sloughs off
- Older cork often sloughs off the outside of the tree
- Older secondary phloem dies and sloughs off as well
- How does the tree then keep producing periderm?
- Tilia americana Multiple periderms example

- Cork oak

- Cork oak

By Cazalla Montijano
- Cork oak
 By Alessandro Vecchi
By Alessandro Vecchi
- Leaf scars and terminal bud scars

- Bark variation: paper birch (Betula papyrifera)

- Bark variation: shagbark hickory (Carya ovata)

- Bark variation: black oak (Quercus velutina)

13.3. Wood
- Wood types
- Softwoods
- Wood from conifers, a group of gymnosperms that includes the Pinaceae, Cupressaceae, Taxaceae and a few others.
- Hardwoods
- Most angiosperm (excluding the monocots!) wood
- Conifer wood: Pinus strobus (Pinaceae)

- Pit pair in Pinus strobus
Margo-torus type pit common in conifer tracheids

- Angiosperm wood: Ulmus americana (Ulmaceeae)

- Ring-porous vs diffuse-porous angiosperm wood
Quercus rubra (Fagaceae)

- Ring-porous vs diffuse-porous angiosperm wood
Liriodendron tulipifera (Magnoliaceae)

- Growth rings in Pinus longaeva

- Cross dating tree rings
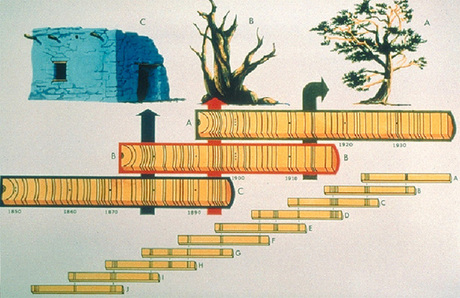
- Branch bases are buried by secondary growth

- loose knots

14. Plant ID workshop #2
15. Phylogenies and taxonomy
15.1. Phylogenies
- Phylogeny
A phylogeny (“evolutionary tree”) is a branching diagram depicting evolutionary relationships among biological entities.

- note B_note
- Phylogenies just depict evolutionary relationships and do not directly imply anything about morphology, traits, etc
- Phylogenies can be rooted (usually how I will depict them in class) or unrooted. A rooted phylogeny implies a direction in which time operates along each branch. An “unrooted” phylogeny means that the true most ancestral node might by any of the internal nodes
- note B_note
- Phylogenetic terms
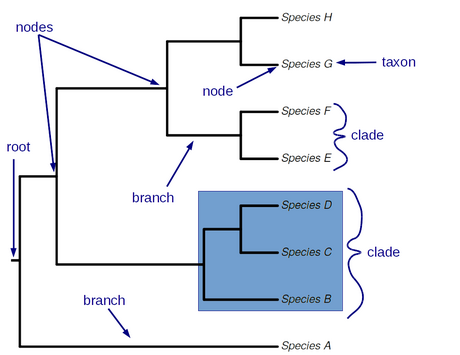
- note B_note
- Phylogeny (the tree)
- branch
- node
- leaf (or tip): corresponds to a taxon
- polytomy (node with more than two descendant branches)
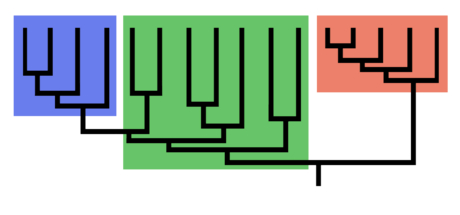
- monophyly / monophyletic (group that contains all the descendants of a common ancestor and no others)
- clade: a monophyletic group. We call two species that form a clade “sister species”
- paraphyly / paraphyletic (non-monophyletic group that excludes descendents of common ancestor)
- note B_note
- Branches can be rotated around nodes without changing relationships
- Modern taxonomies attempt to name only monophyletic groups (clades)
15.2. Taxonomy and Phylogeny
- Naming
Genus and specific epithet
- Categories above genus
- Family
- (end in -aceae). Eg Poaceae
- (no term)
- Others: order, class, division (=phylum), kingdom…
- Monophyly vs non monophyly

- How do we make phylogenies?
We use traits to INFER the phylogeny
- Cladistics
Use shared traits and assume parsimony

- Traits and phylogenies
- Some traits define a monophyletic group. Homologous structures are those that have a common evolutionary origin.
- analogous structures may be superficially similar but arose independently (eg fleshy stems and spines in Cactaceae, Euphorbiaceae, and Asclepidaceae.
- Convergent evolution
Cactaceae Echinocactus texensis

- Convergent evolution:
Euphorbiaceae: Euphorbia tetragona

- Molecular systematics
- Many more more “traits” available (individual loci)
- Can focus on potentially neutral regions of genome
- Chloroplast DNA

16. Origins of the plants
16.1. The Plantae
- Plantae phylogeny

- The green algae
- Chlorophyceae
- Ulvophyceae
- Charophyceae (including Coleochaetophyceae on figure)
- note B_note
Over 17,00 species in three major groups. All have cholroplasts similar to those of the land plants with both cholorophyll a and b and they all store starch in plasmids. Some have firm cell walls, others do not. They vary in cell division. The charophyceae are thought to be most closely related to the land plants. Alternation of generations occurs in some algae, but evolved independently in land plants
- Chlorophyceae

- Chlorophyceae life cycle in Chlamydomonas

- Ulvophyceae

- Some Ulvaceae have alternation of generations

- Charophyceae
Coleochaete

- Chara

- Chara

- Reproduction in stoneworts

- Alternation of generations
16.2. Life on land
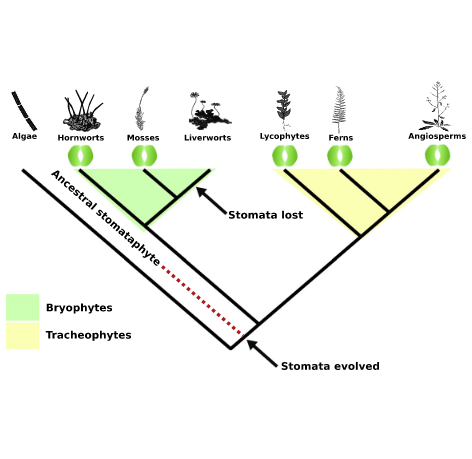
- Plantae phylogeny

- Major groups of land plants
- Nonvascular plants: Bryophytes
Liverworts, mosses, hornworts. All lack vascular tissue.
- Seedless vascular plants
Reproduce via spores (eg lycophytes, ferns, horsetails)
- Seed plants
Includes:
- Non-flowering plants (the gymnosperms = “naked seed”)
- Flowering plants. Angiosperms = “enclosed seed”
- note B_note
If I use a name for a group without quotation marks (eg angiosperms), then that is an accepted monophyletic group. If I use quotes around a name then that is a historical name for a group that is not monophyletic or monophyly is uncertain. There can be disagreement – for example your textbook lists the historical bryophytes (mosses, hornworts, and liverworts) as non monophyletic but recent papers suggest that this group is monophyletic after all. Seed plants are monophyletic
- Nonvascular plants: Bryophytes
- Major events in plant evolution
Dates are approximate!
- 700 mya
- Green algae: first plants with typical modern chloroplasts.
- 475 mya
- Plants invade land
- 400 mya
- Explosion of innovations — roots, overtopping growth (non dichotomous branching), vascular tissue
- 350 mya
- Origin of seeds and wood (bifacial cambium)
- 300-150 mya
- Diversification of the gymnosperm lineages
- 150-50 mya
- Origin and diversification of angiosperms
- 50-10 mya
- Origin and spread of recent groups like the grasses and cacti
- Innovations for life on land
Group = the embryophytes. Origin around 475 MYA
- Retained offspring (hence the name embryophyte)
- Evolution of the cuticle and basic stomata
- Way to protect spores from desiccation (sporopollenin)
- The Bryophytes
They differ from the charophytes (stonewort algae):
- The Bryophytes
They differ from the “standard plant” you have learned about so far.
- Lecture notes B_note
- Main plant generation is haploid
- No true leaves, stems, or roots
- main stem cannot branch
- No xylem or phloem
- note B_note
They have:
- plasmodesmata
- plastids
- sperm are only flagellated cells
- Spores germinate and grow into a protonema (plural protonemata), the juvenile sporophyte
- Protective jacket around gametangia
- growth from apical meristem.
Spores germinate into “Protonemata” first. strandlike gametophyte, single cell wide. THese develope into thallus or more leafy structures.
- Lecture notes B_note
- Relationship with other embryophytes under debate

- Relationship with other embryophytes under debate
17. Bryophytes
17.1. Liverworts
17.2. Mosses
- Reproduction in mosses

- Moss life cycle (example of Bryidae)

- Mosses (=Phylum Bryophyta in book)

- Sphagnum sporophyte spore dispersal

- Sphagnum “leaves” with hyaline cells

- Peat bogs (and fens, mires, etc)

Photo by Merritt Turetsky (@queanofpeat)
- Peat harvesting

- Other moss groups
- granite mosses (Andreaidae)
- “true” mosses (Bryidae)
- Some mosses have a strand of conducting tissue

- Some mosses have a strand of conducting tissue

- Simplified stoma with single guard cell.

- Splash sperm dispersal in Polytrichum

- Spore dispersal in the Bryidae (“true” mosses)

18. Seedless vascular plants
18.1. Vascular plant innovations
- More derived lineages reduce gametophyte generation and sporophyte becomes dominant

- Extant seedless vascular plants
- Origins of vascular tissue: tracheids

- Stele types protostele, syphonostele, eustele

- Stele types protostele, syphonostele, eustele

- Microphylls and megaphylls

- Lecture notes B_note
- microphylls: simple and linear, usually small, but can be multiple inches long (eg Isoetes). Associate with simple protostele of lycophytes
- megaphylls: have venation of vascular tissue. Associated with syphonosteles and Eusteles
- Microsteles probably originated as outgrowths, megaphylss as fused branch systems
- Lecture notes B_note
18.2. Lycophytes
- Isoetes melanospora
.jpg)
- Lycophyte example: Lycopodium

- Selaginella (resurrection plant)

- Selaginella lepidophylla in Big Bend, Texas

- Selaginalla life cycle

- Vascular plant phylogeny

- Innovations: Upright growth and branching
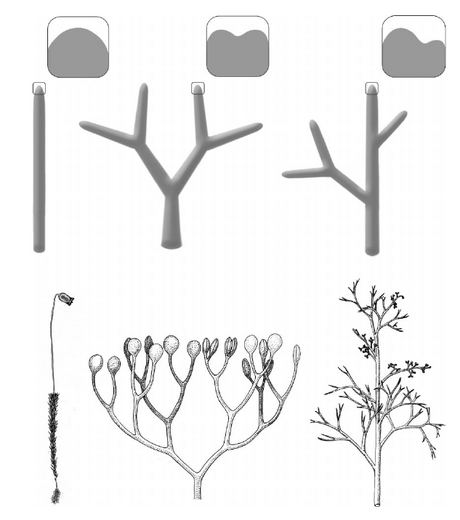
- Note B_note
From Donoghue 2005. Paleobiology 31(sp5):77-93. http://dx.doi.org/10.1666/0094-8373(2005)031[0077:KICASM]2.0.CO;2
FIGURE 4. A comparison of sporophyte branching among early-branching lineages of land plants. In the bryophytic (moss) lineages (left) the sporophyte is unbranched; dichotomous branching evolved at the base of the polysporangiophytes (almost same point as evolution of vascular tissue) (center); overtopping (or pseudo-monopodial growth) evolved at the base of the euphyllophytes (right). Insets at the top represent these differences in branching at the level of the apical meristem. (Drawings at the bottom are from Stewart and Rothwell 1993.)
- Note B_note
- Devonian lycophytes: secondary growth with unifacial cambium
![Donoghue 2005: http://dx.doi.org/10.1666/0094-8373(2005)031[0077:KICASM]2.0.CO;2](figs/Donoghue-2005-Fig6.png)
- Notes B_note
FIGURE 6. A sample of growth forms in extinct lycophytes. Two drawings on the left (from Phillips and DiMichele 1992) show early stages in the life cycle—establishment of the stigmarian “root” system with possibly photosynthetic “rootlets” prior to rapid stem elongation. Three drawings on the right (from Stewart and Rothwell 1993) show reconstructed forms of the determinate stems (not drawn to the same scale); from left to right: Sigillaria, Pleuromeia, and Lepidodendron.
- Notes B_note
- Unifacial vs bifacial cambium
![Donoghue 2005: http://dx.doi.org/10.1666/0094-8373(2005)031[0077:KICASM]2.0.CO;2](figs/Donoghue-2005-Fig5.png)
- Note B_note
From Donoghue 2005. Paleobiology 31:77-93. http://dx.doi.org/10.1666/0094-8373(2005)031[0077:KICASM]2.0.CO;2
FIGURE 5. Differences between the bifacial cambium in the lignophyte lineage (including seed plants) and the unifacial cambium found in extinct tree lycophytes (e.g., Lepidodendron). The bifacial cambium produces both secondary xylem and secondary phloem, and the cambial initials are able to divide both periclinally (producing cells that differentiate in secondary tissues) and anticlinally (producing new cambial initials). The unifacial cambium produced only secondary xylem and the cambial initials divided only periclinally, limiting expansion of the cambial cylinder and the production of wood. These seemingly minor differences translated into major differences in evolutionary flexibility and “success”.
- Note B_note
18.3. Monilophytes
- Wisk fern (Psilotum)

- fern example: Cyathea

- fern example: Adiantum

- Adiantum rhyzome

- Adiantum rhyzome

- Fern sori in Polypodium

- Fern sori in Pteridium aquilinum (Bracken fern)

- Fern sori in Polystichum

- Horsetails (Equisetum)

- Equisetum at White River rest stop

- Equisetum strobili

- Equisetum spores

- Equisetum stem with hollow pith

18.4. Age of the tracheophytes
- When did these innovations occur?

- “Trees” of the Devonian and Carboniferous
![Donoghue 2005: http://dx.doi.org/10.1666/0094-8373(2005)031[0077:KICASM]2.0.CO;2](figs/Donoghue-2005-Fig7.png)
- Notes B_note
FIGURE 7. Diversity of form among extinct treelike plants from the Devonian and Carboniferous (not drawn to the same scale). From left to right: Archaeopteris (an early lignophyte); Calamites (an equisetophyte); Psaronius (a marattialean “fern”), in which the trunk was formed by a mantle of adventitious roots; Tempskya (a filicalean “fern”), in which the trunk was formed by numerous smaller stems embedded in a tangle of adventitious roots.
- Notes B_note
- Early secondary growth and coal formation

- Notes B_note
- Originally thought there was a delay in ligin degradation evolution but now we believe simply was climatic conditions suitable for no decomposition. https://www.pnas.org/doi/10.1073/pnas.1517943113
- Notes B_note
19. Gymnosperms
19.1. Seed plant phylogeny and innovations
- Phylogeny

- Key innovations
- Evolution of the ovule (where seed is formed)
- Megaspore retained inside megasporangium (called nucellus in seed plants)
- Reduction to 1 megaspore mother cell per megasporangium and only one surviving megaspore.
- Female gametophyte intirely WITHIN megaspore and after fertilization, embryo also entirely within megasporangium
- Integument envelopes megasporangium except for one opening
- Ovule

- Notes B_note
- Extreme form of heterospory
- Megaspores retained in megasporangium (= nucellus)
- One megaspore mother cell per megasporangium
- Unequal division leads to one surviving megaspore daughter cell
- Female gametophyte forms inside megaspore
- embryo held in this female gametophyte
- Megasporangium enclosed except for opening at end: micropyle
- Conifer seeds contain cells from three generations of the tree. The nutritive tissues inside the seed are the haploid body cells of the female gametophyte. The seed also contains the developing diploid sporophyte. The the tough and protective seed coat is formed from the diploid cells of the parent sporophyte.
- Notes B_note
- Pollen
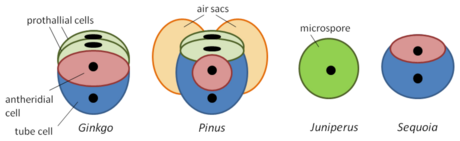
19.2. Pinophyta
- Pinophyta (Pines, firs, spruce)

- Pinus ponderosa

- Spruce and the Alaskan pipeline

- Lodgepole pine (Pinus contorta) cones

- Note B_note
- Occur on separate cones on same tree
- This species is common at high elevations across the western US. Some populations have serotinous cones that open following wildfire. This tree is often one of the first conifers to colonize wet meadows as it can withstand more waterlogged soil than can many other trees.
- Note B_note
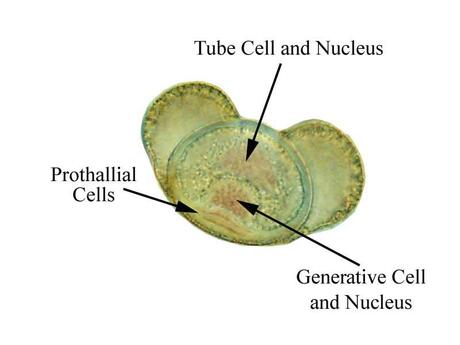
- Pine pollen grain: microgametophyte

- Pine pollen grain
- Pine megaspores in female cone

- Pine megaspores in female cone

- Notes B_note
- Megasporocyte is mother cell of megagametophyte
- No division of this cell takes place until AFTER pollination. This works out because it takes about 15 months for pollen tube to grow down and sperm nuclei to travel to egg!
- Note no antheridia – male gametophyte has almost no structures.
- See also [[https://en.wikipedia.org/wiki/Conifercone#/media/File:Pinussylvestrisfemalestrobilusandconeen.svg][
]]
- Notes B_note
- Pine life cycle 1

- Pine life cycle 2

- Pine life cycle 3

- Pine life cycle 4

- Pine life cycle 5

- Pine life cycle 6

19.3. Gnetophyta
- Gnetophyta: Ephedra sp in Arizona

- Note B_note
Three genera in this division: Ephedra, Gnetum, and Welwitschia. I took this photo of an Ephedra species in Walnut Canyon, Arizona near Flagstaff. But Ephedra antisyphilitica occurs right around Lubbock and we saw it on our field trip.
Gnetum is found in tropics around the globe. Both Welwitschia and Gnetum havea type of double fertilziation but it forms a second embryo that aborts. As in other gymnosperms, the seed is nourished by gametophyte tissue, not an endosperm.
- Note B_note
- Gnetophyta: Welwitschia

- Note B_note
Photo: Thomas Schoch - http://www.retas.de/thomas/travel/namibia2003/index.html, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=650590
Welwitschia is named after the Austrian botanist and doctor Friedrich Welwitsch who discovered the plant in 1859 in present-day Angola. Welwitsch was so overwhelmed by the plant that he, “could do nothing but kneel down and gaze at it, half in fear lest a touch should prove it a figment of the imagination.”
Dr. Welwitsch abandoned medicine to study botany full time in 1839 and has life improved as a consequence.
- Note B_note
19.4. Cuppressophyta
19.5. Ginkgo and Cycads
- Ginkgo biloba

- Ginkgo biloba male cones

- Note B_note
Native to China but planted all over the world. The only species in the Division Ginkgophyta. Has good clonal (asexual) reproduction from basal buds or roots that form on damaged/drooping branches.
The species is important in traditional Chinese medicine. The species is dioeceous.
The sperm has multiple flagella and swims to the ovule after pollen lands on the stalk tip. See https://www.arboretum.harvard.edu/the-swimming-of-the-ginkgo-sperm/
- Note B_note
- Ginkgo biloba female tree with ovules

- Ginkgo fertilization

- Ginkgo seeds and sperm
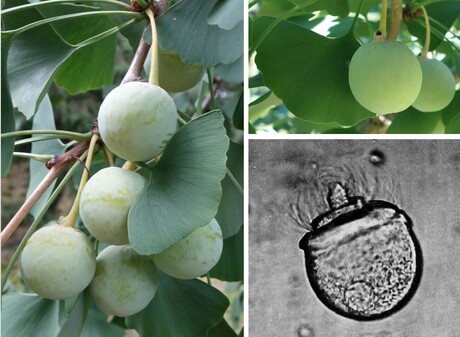
- Cycads (Cycadophyta)

Photo: Muhammad Mahdi Karim
- Cycads in Australia: Cycas brunnea

Alastair Freeman, 2010
20. Angiosperms
20.1. Overview
20.2. Angiosperm life cycle
- Anthers

- microsporocytes (diploid) in center,
- tapetum is nutrient rich tissue around.
- Other tissue forms wall of pollen sac
- Anthers

- Pollen (the microgametophyte)

- Notes B_note
- In some species, generative cell divides before pollen is dispersed, that results in three-celled pollen grain.
- Pollen has inner wall (intine) and outer wall (exine). Thin places or holes in exine are sites for pollen tube initiation.
- Sporopollenin coat is synthesized by the anther tapetum tissue and is a polymer of carotenoids
- Protects pollen from UV, dehydration, pathogens
- An additional coat is added by tepetum (pollen coat).
- Notes B_note
- Pollen grain example: Aesculus hippocastanum (Sapindaceae)

- Pollen grain example: Cucurbita pepo (Cucurbitaceae)

- Ovule structure

- Formation of ovule

- Notes B_note
- Most common megasporogenesis (a) involves:
- diploid megasporocyte undergoes meiosis and results in 4 haploid megaspores. 3 disintegrate.
- The megaspore nucleus then divides mitotically three times resulting in eight nuclei arranged in two groups of four.
- Two migrate to center to form polar nuclei
- cell walls form around three antipodals and also around egg and two synergids by egg nucleus. Result is 7 cells, 8 nuclei.
- fitellaria type no cell walls during meiosis step.
- oenethera type: ancestral. four cells, 4 nuclei at maturity.
- Others have 8 cells, nine nuclei at maturity.
- Notes B_note
- Pollen tube

- Angiosperm life cycle: soybean

- Angiosperm life cycle: soybean

- Angiosperm life cycle: soybean

- Angiosperm life cycle: soybean

- Angiosperm life cycle: soybean

21. Angiosperm Flowers
21.1. Floral variation
- Inflorescences

- Inflorescences

- Placentation

- Ovary position

- Inferior ovary example: Malus domestica (apple, Rosaceae)

- Inferior ovary example: Malus domestica (apple, Rosaceae)

- Floral symmetry
- Radial (actinomorphic, regular)
- Bilateral (zygomorphic, irregular)
- Flowers: perfect or imperfect
- Perfect flower
- functional stamens and functional stigmas
- Staminate flower
- functional stamens only
- Carpellate flower
- functional stigmas
- Plant sexes:
which means that plants have several combinations
- Hermaphroditism
- perfect flowers only
- Monoecy
- staminate and carpellate flowers on same plant
- Dioecy
- Separate male and female individuals
- A dioecious plant: Cannabis sativa

21.2. Evolution of the angiosperms
- Angiosperm phylogeny

- Amborella trichopoda

- Amborella trichopoda

- Notes B_note
- Found only in rain forests of New Caledonia
- Sister to all other angiosperms
- That means it separated from them about 120-140 million years ago! It is the tip of a very long branch and we lack fossil information on its relatives.
- It does have some archaic features, however:
- Wood without vessels, only tracheids
- flowers with tepals, stamens, carpels, spirally arranged in indefinite number
- Unsealed carpels
- But it has unisexual flowers and one seed fruits which different from what we believe early angiosperms had
- Notes B_note
- Nymphaceae

- Austrobaileya scandens

- Notes B_note
- There are other Austrobaileyales including star anise (Illicium anisatum)
- These early groups all show the third megagametophyte patterns where there is a single polar nucleus and therefore a diploid endosperm.
- ancestral traits: tepals, stamens with innermost sterile (Separate stamens from carpels) then many carpels. 12-15 carpels each with 8 to 12 ovules
- Notes B_note
- Magnoliid: Liriodendron tulipifera
![[[tulip tree image by Bruce Marlin CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0)][<a href="https://upload.wikimedia.org/wikipedia/commons/0/05/Liriodendron_tulipifera.jpg">Link</a>]]](figs/Liriodendron_tulipifera.jpg)
- Magnoliid: Magnolia grandiflora

- Trends in Angiosperm floral evolution
- Many and indefinite flower parts to few and definite
- Axis shortened and floral parts fused
- Inferior ovary in more derived groups
- Radial symmetry to bilateral symmetry
- Lonicera (Caprifoliaceae, in the Asterid group)

- Gossypoium (Cotton, Malvaceae)

- Asteraceae: Helianthus annus

- Disk and ray flowers

- Cirsium pastoris (Asteraceae)

- Agoseris (Asteraceae)

- Orchidaceae

- Platanthera limosa
21.3. Animals and flowers
- Coevolution with animals

- Rodent pollination in Proteaceae

- Rodent pollination
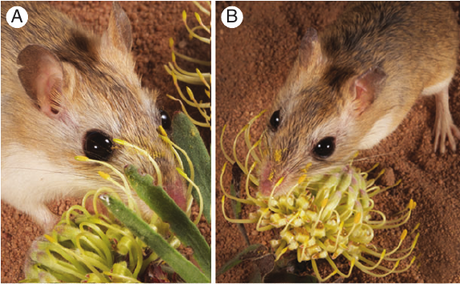
- Note B_note
Johnson, C. M., & Pauw, A. (2014). Adaptation for rodent pollination in Leucospermum arenarium (Proteaceae) despite rapid pollen loss during grooming. Annals of botany, 113(6), 931–938. https://doi.org/10.1093/aob/mcu015, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3997634/
See also a nice summary at https://www.indefenseofplants.com/blog/2018/9/24/rodents-as-pollinators
- Note B_note
- Fly pollination in Stapelia schinzii (Apocynaceae)

- Insect vs wind pollination

- Heterostyly in Lythrum salicaria (Primulaceae)

- Note B_note
See Eckert et al 1996: https://www.nature.com/articles/hdy1996185
- Note B_note
- Distribution of types becomes more even over time

- Colors not aimed at us

- Pollination through deception

- Pollination through deception

- Flower coloration
22. Fruits and seeds
22.1. Fruit types
- The fruit
Mature ovary and possible accessory tissue
- Major fleshy fruit types
- Berry. Multiple seeds
- Drupe. One seeded.
- Pome. Compound inferior ovary
- Berry: Eg Solanum lycospersicum (Solanaceae)

- Drupe: Eg Prunus spp.

- Pome: Pyrus spp.

- Major dry fruit types
- Capsule and silique

- Legume: Bean (Phaseolus spp)

- Loculicidal capsule on Yucca

- Yucca shidigera

- Yucca moth pollination

- Yucca moth pollination

- Achene, samara, cypsela, and caryopsis
- BMCOL

- BMCOL

- Lecture notes B_note
- Achene and samara and cypsela: pericarp connected to seed coat just at funiculus so it can peel off
- cypsela is term for the achene-lie fruit of a asteraceae (inferior ovary)
- samara is a winged achene
- caryopsis is a grain: grasses. pericarp fused to seed coat over entire surface.
- BMCOL
- Nut: hazelnut, Corylus (Betulaceae)

- Pecan, Carya illinoinensis (Juglandaceae)
A drupe!

- Nutmeg and mace, Myristica fragrans (Myristacaceae – a Magnoliid)

- Myristica fragrans

- Multiple carpels
- Lecture notes B_note
- Can lead to simple fruit like a pone, or a berry
- Aggregate fruit: multiple ovaries in single flower. Blackberry fruit of druplets
- Aggregate accessory fruit: Strawberry. Achenes on fleshy receptacle
- Multiple fruit: Multiple flowers merge. Pineapple is example. The “cones” of some proteaceaae
- Lecture notes B_note
23. Photosynthesis: light reactions
23.1. Overview
- Questions:
- What is the most abundant protein on the planet?
- Why is the global atmospheric CO2 level higher in January and lower in June?
- Can plants photosynthesize in the dark?
- Most important biochemical reaction on the planet
In its current form (O2 producing), evolved nearly 3 billion years ago in cyanobacteria.
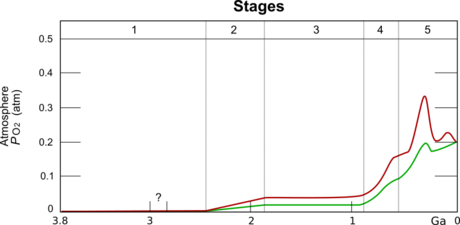
- The great oxygenation event
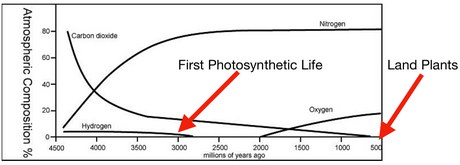
- Global atmospheric C02
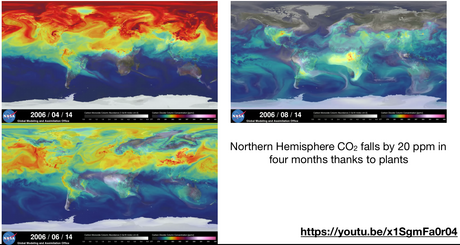
- CO2 over time
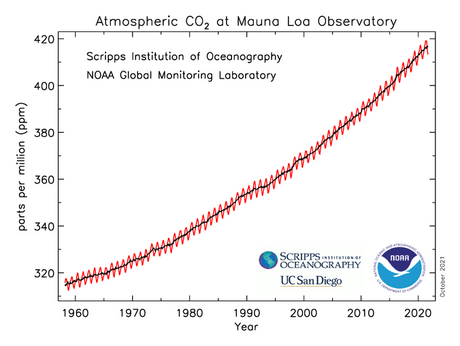
- CO2 over time
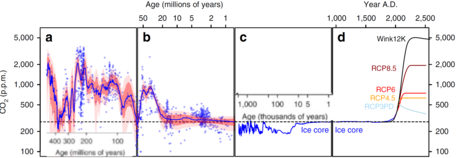
- Lecture notes B_note
Fig 4 from Foster, G., Royer, D. & Lunt, D. Future climate forcing potentially without precedent in the last 420 million years. Nat Commun 8, 14845 (2017). https://doi.org/10.1038
- Lecture notes B_note
- Sunlight to carbohydrate
- Photosynthesis vs respiration
- Where does the oxygen come from?
- Less simple equation
- Chloroplasts
 u
u
- Chloroplast structure

- Two linked reactions

- Short term energy storage: ATP and NADPH
- Longer term energy: glucose
- Harvesting light energy
First step is to produce a hydrogen (H+) diffusion gradient by splitting water into O\(_2\) and hydrogen (protons)
23.2. Light reactions
- Electromagnetic spectrum
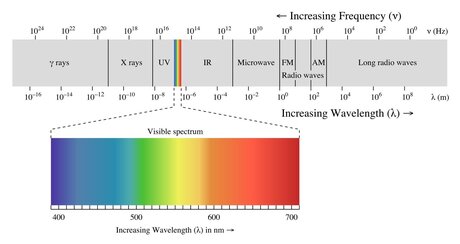
- How to get useful energy from light?
- Absorption
Two major pigment classes in plant leaves: Chlorophylls (a and b) and Carotenoids (carotenes and xanthophylls)

- Chlorophyll a

- Photosystems
Chlorophyll molecules work together in groups
- Photoexcitation

- Two types of reaction centers:
- Photosystem II
- Photosystem I
These photosystems work together: electron output of II is electron input to I. They are linked by a enzyme complex: Cytochrome
- note B_note
Photosynthesis increases dramatically when cells are exposed to both red and far-red light simultaneously. The fact that photosynthetic rate is higher under both light sources than the sum of the rates under each light source individually is known as the “enhancement effect”. This indicates a coupled set of processes.
- Z-Scheme

- Note B_note
Here is a video describing the light reactions: https://www.youtube.com/watch?v=hj_WKgnL6MI
- Note B_note
- Electron transport chain
- Electrons captured by electron acceptors in PSII and used to power proton pump
- Electrons excited again in PSI and eventually transferred to NADP+
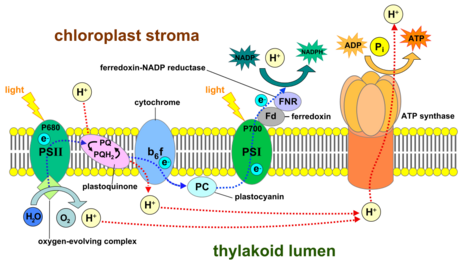
- Review: steps in storing chemical energy
- Sunlight excites electrons in reaction centers
- Energy in excited electrons used to split water to release protons (H+) and O\(_2\)
- This creates a hydrogen ion (H+) diffusion gradient.
- Diffusing H+ powers ATP production. NADPH is produced in second electron transport chain in PSI
- Cyclic photophosphorylation
PSI only

24. Photosynthesis: Carbon fixation
- Calvin Cycle
- The energy transformation of the light-dependent reactions
- Which itself involves linked reactions in PS II and PS I
- The carbon dioxide reduction of the Calvin cycle
- The energy transformation of the light-dependent reactions
- The Calvin Cycle
Benson and Calvin 1950

- Molecules to know
- Chlorophyll: Plant pigment that absorbs light
- Cytochrome: Enzyme that pumps protons against a gradient
- ATP / ADP: Energy storing molecule
- NADPH / NADP+ : electron shuttle
- ATP-synthase: Enzyme that creates ATP (phosphorylizes ADP), powered by proton gradient
- RuBisCO: Enzyme that fixes carbon dioxide into organic carbon
- 3PGA: first carbon product fixed in cycle. 3-phosphoglycerate.
- G3P or “PGAL”: 3-carbon sugar that is the output of the Calvin Cycle. glyceraldehyde 3-phosphate
24.1. Rubisco and photorespiration
- Rubisco
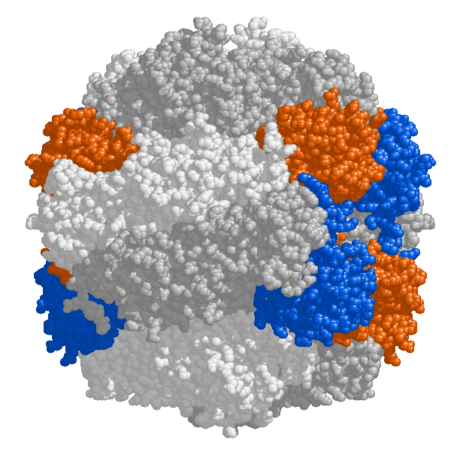
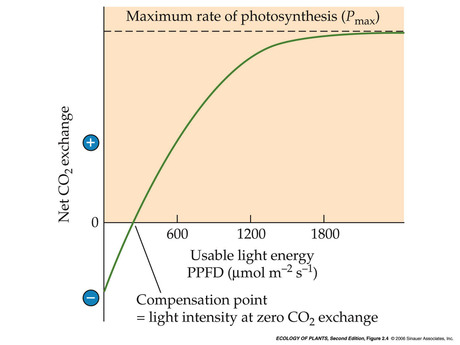
- Limitations on photosynthesis
- Carboxylase and Oxygenase
Rubisco is also an oxygenase — when O2 concentration is high compared to CO2, rubisco binds to RuBP and O2
- Content of atmosphere
This is the problem:
- 78.1% N2
- 20.9% O2
- 0.9% Ar
- 0.04% CO2
- 0.002% Ne
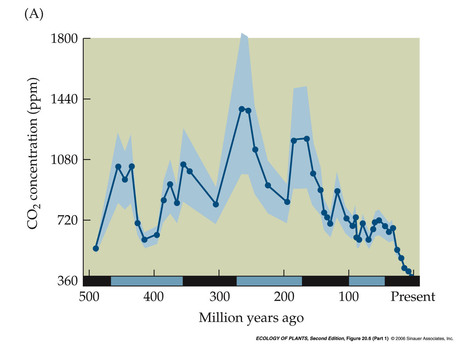
- Atmospheric CO2 concentration through time
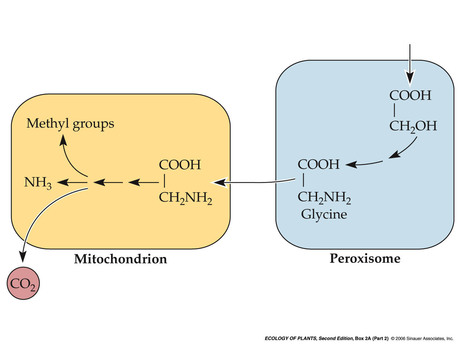
- Photorespiration: chloroplast
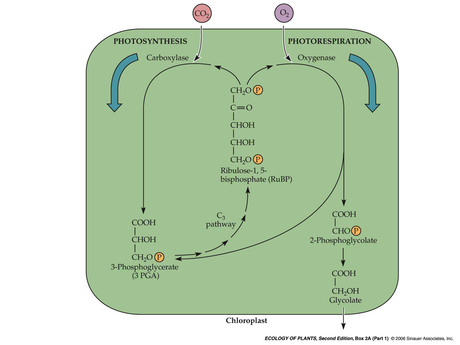
- Photorespiration: perixosome and mitochondria
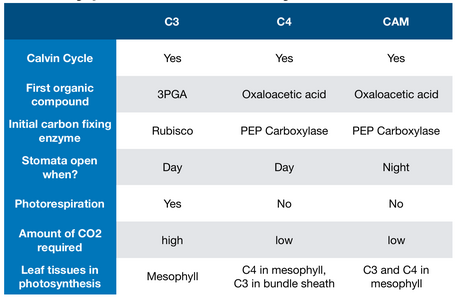
25. Photosynthesis: C4 and CAM
25.1. C4 Photosynthesis
- When is photorespiration a problem?
- When atmospheric CO2 is low
- When internal CO2 is low (stomata narrowed to prevent water loss)
- When light and temperature are higher
- C4

Gurevitch et al 2004
- Anatomy of a C3 leaf
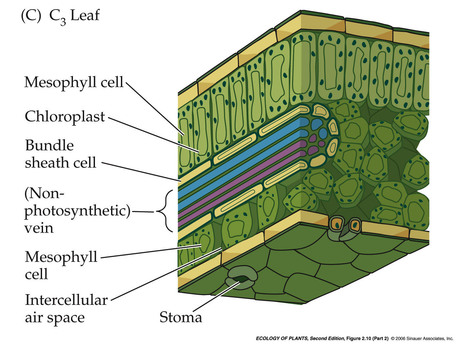
Gurevitch et al 2004
- C4 leaf: spatial separation of light rx and carbon aquisition
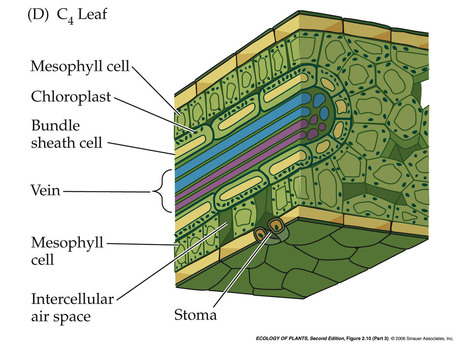
- C4 Plants: Sugarcane

- C4 Plants: Maize

- C4 Plants: Blue grama

Copyright Curtis Clark
- Advantages of C4:
- Higher maximum rates of photosynthesis
- Higher temperature optimum
- Higher water use efficiency
- Higher N use efficiency
- C4 CO2 response
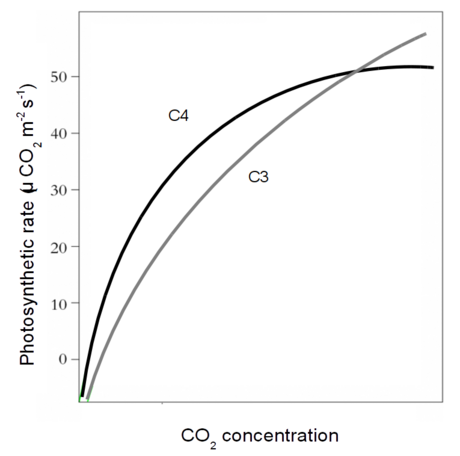
- C4 photosynthesis
- evolved up to 50 times independently
- present in 19 angiosperm families
- only 3% of angiosperm species are C4
- But they account for 20% of earth’s total primary production
- Atmospheric CO2 concentration through time

- When did C4 evolve?
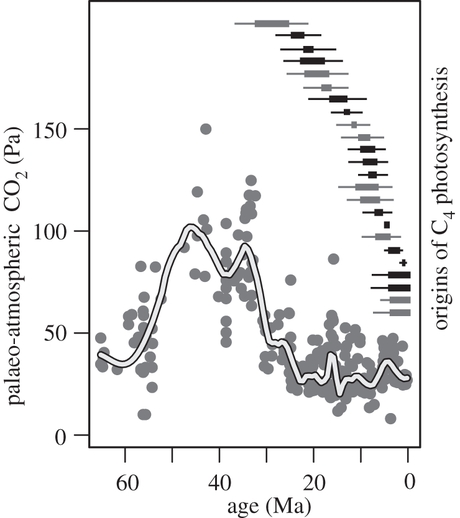
Figure: Osborne and Sack 2012
- Distribution of C4 grasses in North America
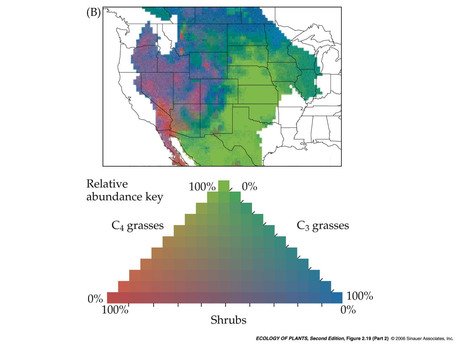
Figure: Gurevitch et al 2004
- When does C3 outperform C4?
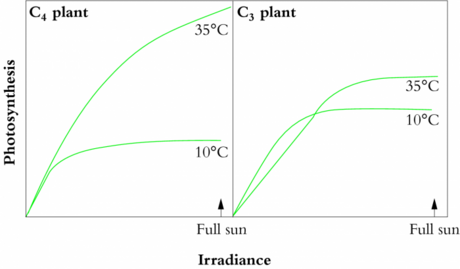
- Where does one find C4 plants?
- Warm climates with some productivity (not too dry).
- But not so productive that trees overtop grasses!
- So landscapes like the southern High Plains where there is a warm and reasonably wet summer, but rainfall variability or total rainfall limits trees
- Or semi-tropical grasslands where fire limits trees
- Carbon isotopes useful for detecting past vegetation from paleosoils
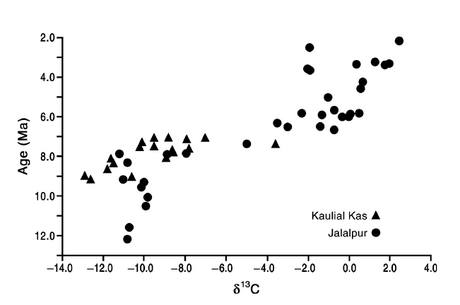
- Subtropical grasslands

- Spread of C4 occured well after evolution of C4!
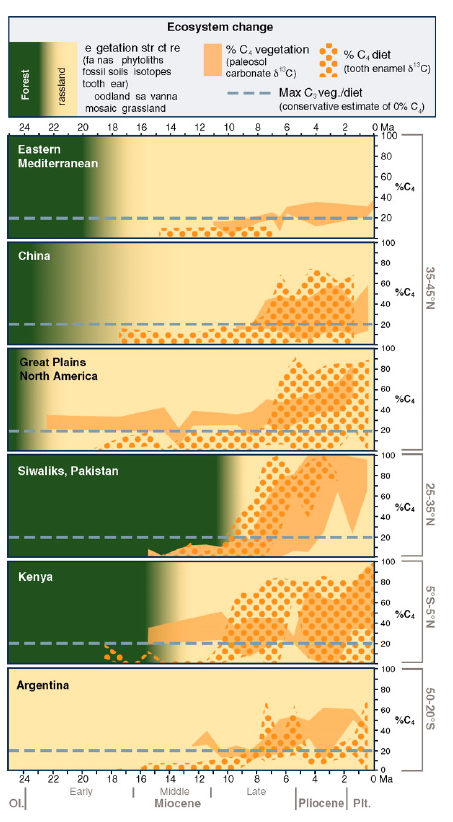
- What controls C4 grass distribution today?
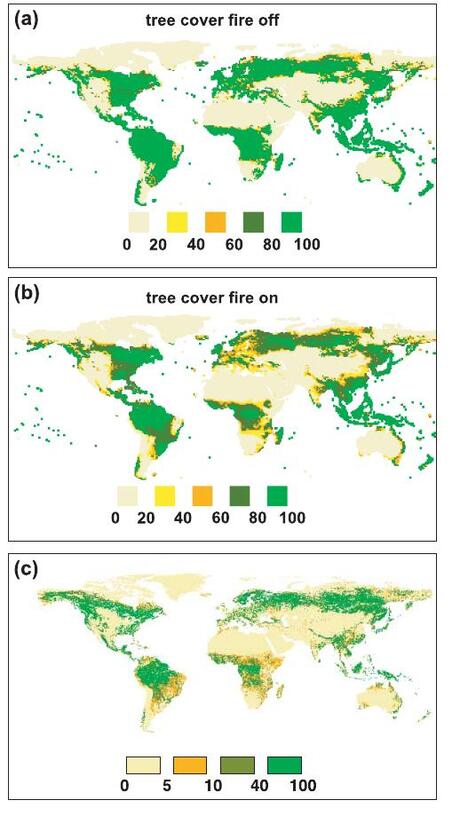
- What happened in the Miocene that allowed expansion of C4 vegetation?
- Increased aridity
- Increased seasonality
- Increased fire (charcoal evidence)
25.2. CAM Photosynthesis
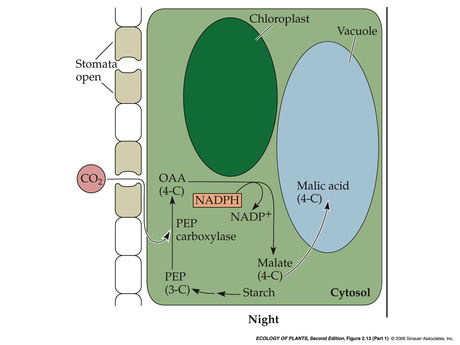
- Crassulacean Acid Metabolism (CAM)
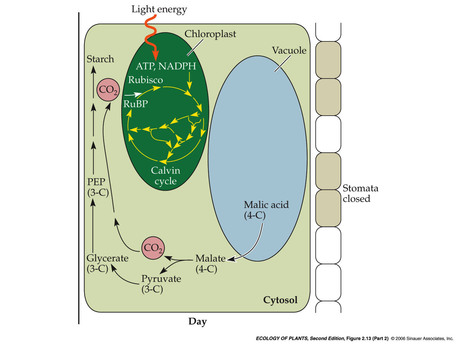
- Temporal separation: night
- Temporal separation: day
- Where do you find CAM plants?
Over 30,000 species
- Desert succulents (Cactaceae, Agavaceae, Euphorbiaceae)
- Tropical epiphytes (Bromeliads, some orchids)
- Some aquatic plants (??!!)
- CAM plants: Euphorbia tetragona

- CAM plants: Cactaceae (Echinocactus texensis)

- CAM plants: Agave neomexicana

- CAM plants: Bromeliads
Tillandsia in Florida

photo: Hans Hillewaert
- CAM plants: Aquatic? Isoetes
.jpg)
Figure: Wikimedia commons AHR 2013
- Note
CAM photosynthesis has also been found in a few aquatic plants. What value would it serve for such plants? See http://www.jstor.org/stable/4354319
- Note
- Types of photosynthesis
26. Water movement 1
26.1. Key Concepts and overview
- Transpiration

- Plants balance root water acquisition with transpiration
- Nearly all water loss is to the atmosphere
- Nearly all water gain is via roots
- All other sources negligible (metabolic water gain, loss in photosynthetic reaction)
- Stomata

- Water is pulled up the plant through xylem tracheids and vessels
- Water moves up the plant and down a potential energy gradient
- Major forces moving water
- Water can be under tension (negative pressure)
Hydrogen bonds allow cohesion of water molecules to one another and adhesion to charged surfaces.
26.2. Water in air
- Water in air
Water vapor is one component of air
- Water vapor pressure
- Denoted \(e\). Partial pressure of water vapor in atmosphere. Measured in pressure units (eg KPa) or in relative volume (cm3 / m3). This is “absolute humidity”.
- Saturated vapor pressure
- Denoted \(e_0\). Partial vapor pressure when liquid and gas phase of water are at equilibrium. Temperature dependent.
- VPD and RH
- Vapor Pressure Deficit (VPD) and Relative Humidity (RH) are two ways of describing the amount of water in air relative to the saturation vapor pressure.
- Saturation vapor pressure
- Why is water pulled from leaves?
- Transpiration
Results from water moving from high concentration (inside leaf) to low (dry air)
- Vapor pressure deficit (VPD):: \(e_0 - e\). Difference between saturated vapor pressure and actual vapor pressure. Depends on actual amount of water in air and on temperature
- Relative humidity (RH):: \(\frac{e}{e_0} \times 100\)
Whenever VPD is non zero (when RH < 100%), then the atmosphere can potentially pull water from leaves!
- Transpiration
- Saturation vapor pressure and VPD
26.3. Water potential
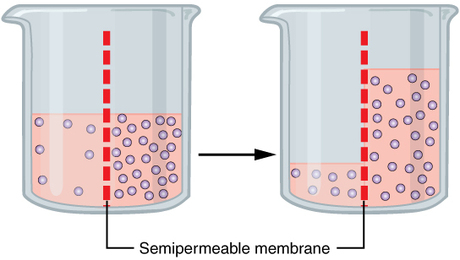
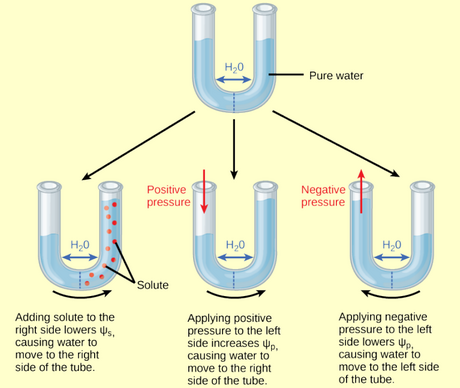
- Water potential
- Water potential is sum of components
Ψ = Ψosmotic + Ψmatric + Ψvapor + Ψpressure + Ψgravity
- \(\Psi\) is in units of pressure
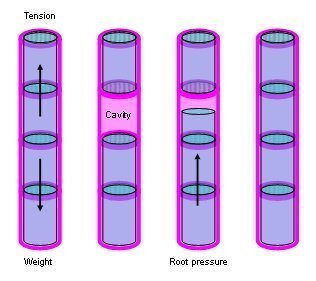
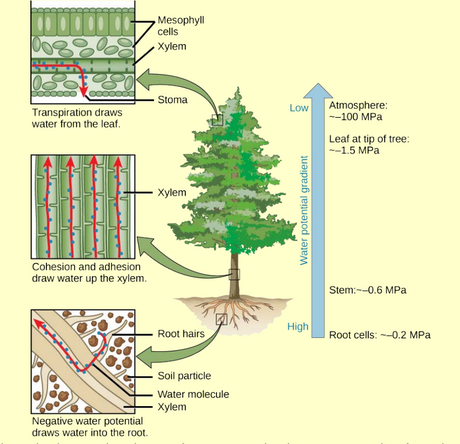
- Cohesion-tension theory of water transport
- Note B_note
We usually us megapascals (MPa) as the units of water potential. One MPa is about 145 pounds per square inch of pressure. This means that a well watered plant with a turgid leaf may have leaf mesophyll cells at around 210 pounds per square inch of pressure (\(\Psi_{pressure}\) = 1.5 MPa). A desert shrub during drought might have xylem vessels at -7 MPa, which is just over 1000 lb/in\(^2\) !
- Note B_note
- Water potential from soil through plant
- Soil to plant
- \(\Psi_{osmotic}\) of root cells must be lower than \(\Psi_{matric}\) of soil water to draw water into roots.
- Within the plant
- \(\Psi\) at any point \(\Psi{}_{solutes} + \Psi{}_{pressure}\)
- Root cells to xylem vessels
- \(\Psi_{pressure}\) of xylem must be lower than \(\Psi\) of root cells
- Xylem vessels to leaf cells
- \(\Psi_{osmotic}\) of leaf cells must be low to draw water from xylem
- Transpiration
- Leaf cells to atmosphere is driven by very low (NEGATIVE) \(\Psi\). Eg -100 MPa.
- Turgor pressure
When pressure inside the cell (turgor pressure) increases, the cell wall pressure is induced. A cell that is firm is turgid
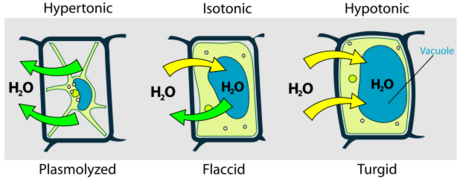
- A turgid cell can still have negative water potential
- Loss of turgor = Wilting

- Measuring water potential

- “Pressure Bomb”

27. Water movement 2
27.1. Water uptake and soil water
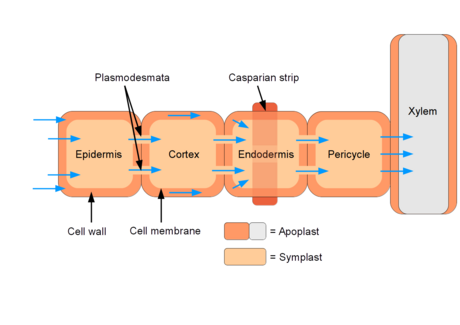
- Symplast and apoplast
- Remember that xylem tissue is primarily dead cells
- And live plant cells have plasmodesmata
- Water entry into roots
Water must get from soil through epidermis and cortex tissue to vascular tissue
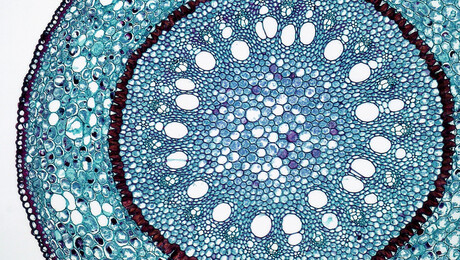
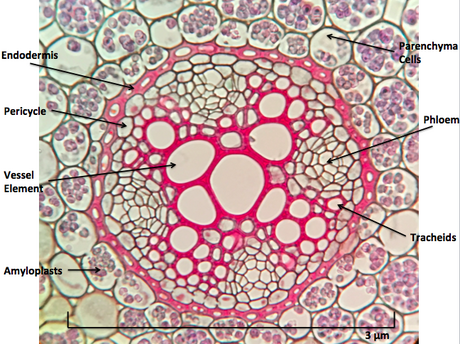
- Root endodermis
- The Casparian strip
Narrow band of wax on endodermal cells. Composed of suberin.
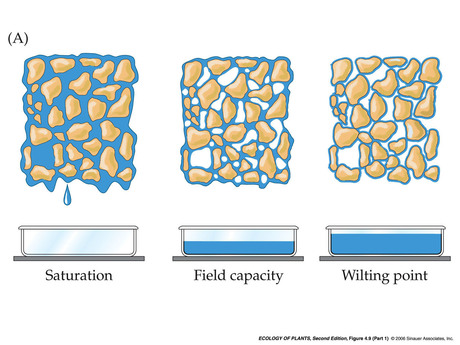
- Soil water: matric forces
- Salt scalding

- Salt scalding

- Predawn vs midday water potential
- During the night, VPD drops and stomata are closed (for C3/C4)
- Therefore, flow stops and Ψ equilibrates
- Predawn water potential then indicates something about soil water potential
- Example: Mesquite

- Example: Juniper

- Water potential question:
During late summer you measure leaf water potential pre-dawn in a juniper and mesquite growing side by side. You find that the predawn Ψ for the juniper is -5 MPa and for the mesquite it is -2 MPa.
27.2. Xylem and cavitation
27.3. Plant adaptations to drought
- Whole plant adaptations
- Drought avoidance whole plant (desert annuals)
- Drought avoidance through phenology: drought deciduous (deserts and Mediterranean shrublands)
- Drought tolerance:
- Desiccation avoidance: High root-shoot ratios, succulents
- Desiccation tolerance requires specific xylem anatomy)
- Whole plant adaptation examples
\begin{overlayarea}{10cm}{7cm} \centering \includegraphics<1>[height=7cm]{figs/namaqualand.jpg} \includegraphics<2>[height=7cm]{figs/amboy.jpg} \includegraphics<3>[height=7cm]{figs/ocotillo.jpg} \includegraphics<4>[height=7cm]{figs/buckeye.jpg} \includegraphics<5>[height=7cm]{figs/Velvet_mesquite.jpg} \includegraphics<6>[height=7cm]{figs/horse_crippler.jpg} \end{overlayarea} - Anatomical adaptations
- Desiccation avoidance:
- Stomatal number, size and arrangement, control
- Isobilateral leaves (symmetrical internal architecture, amphistomatous: stomata on both sides of leaf
- Waxy cuticle or hairs
- Curl or fold leaves
- Desiccation tolerance:
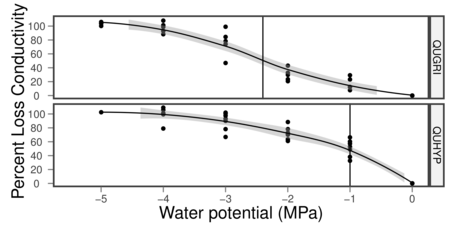
- Extreme xylem resistance to cavitiation
- Narrow vessels?
- Ability to resprout following dieback
- Desiccation avoidance:
- Nerium oleander

- Anatomical adaptation example: stomatal crypts

27.4. Drought in West Texas
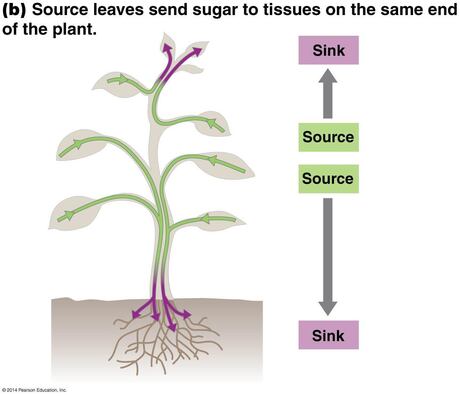
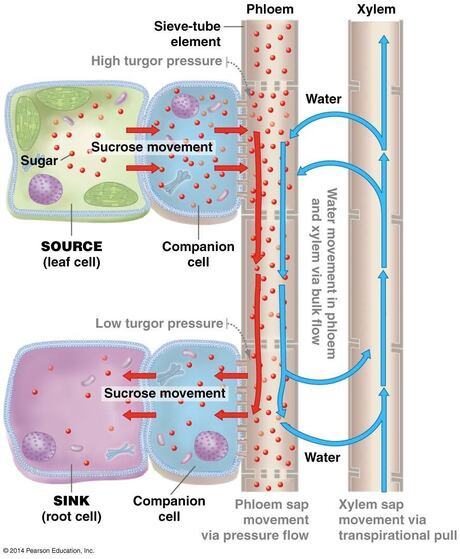
28. Phloem and Introduction to plant hormones
28.1. Water and sugar
28.2. Plant hormones
- Hormones
- Greek “hormon” means to stimulate
- Chemicals that are active in small quantitied
- Induce a response to control a physiological event — regulate.
- Major hormone groups
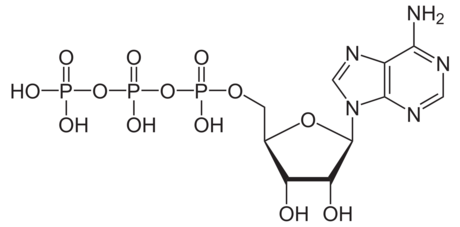
- Auxins
- control apical dominance, differentiation of tissues, abscission, flowering, fruit development…
- Cytokinins
- Produced mostly in roots, travels through xylem to shoot, con control apical dominance and leaf senescence
- Ethylene
- Gas produced in multiple tissues. Huge effect on fruit ripening.
- Abscisic acid
- Made in leaves and roots in response to stress. Important in stomatal closure, also important in many dormancy mechanisms.
- Gibberelins
- Control cell elongation, seed germination, stimulation of flowering
- Brassinosteroids
- Act locally near point of synthesis. Wide range of effects
- Auxins: polar transport

- Lecture notes B_note
Charles Darwin performed experiments published in 1881. Power of movement in plants. Grass seedlings. bent toward light only if tip (apical meristem) was exposed to light. But bending occurred in cell elongation further down the coleoptile.
In 1926 Frits Went named the substance “Auxin” (Indole-3-acetic acid).
- Lecture notes B_note
- Auxins
- Produced in shoot, travels polarly down towards roots
- Indirectly inhibits axilary bud growth
- Promotes lateral root formation
- promotes expansion of vascular cambium in woody plants
- Apical dominance and auxins

- Auxins in fruit
Produced by developing seeds


- Cytokinins (example, Nicotiana tissue on agar)

- Ethylene
- Fruit ripening
- shoot growth behavior changes in response to stress
29. Control of growth
29.1. Tropisms
- Gravitropism

- Sensed via amyloplasts settling to lower side of cell
- In shoots: in statocyte cells in the starch sheath (innermost cortical cells)
- In roots: rootcap — cytokinin produced on lower side of root tip and that inhibits cell elongation.
- Root tip with free cytokinin in blue

- Other tropisms
- hydrotropisms in roots
- thigmatropism (eg pea tendrils).
29.2. Timing of physiology
- Circadian rhythms
- 24-hour internal clocks continually entrained (reset) by environment (eg day/night)
- Control flower opening and closing (eg Datura)
- Leaf movement
- Control sensitivity to particular hormones (gating)
http://plantsinmotion.bio.indiana.edu/plantmotion/movements/leafmovements/clocks.html
- Photoperiodism
Day length controls flowering in many plants

- Measuring day length
Plants don’t measure day length, they measure darkness!
Karl Hamner and James Bonner in 1938: cocklebur, a short day plant
- Found that a single leaf was enough to sense day length.
- discovered a single minute of light during the night STOPPED the flowering response of the short day.
- Lettuce seed experiments with red and far red light
Lactuca sativa seeds need light to germinate. Red light works best (660 nm). Far red light can inhibit germination even better than darkness
- Phytochrome: two forms

- Phytochrome: two forms

- Phytochrome and shade/neighbor detection

- Day length and latitude
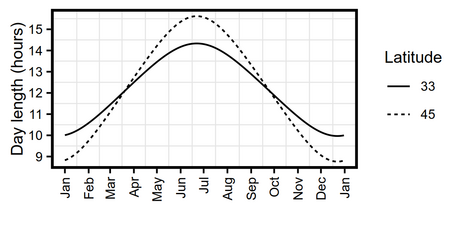
- Vernalization and stratification
- many plants will only flower if the plant is first exposed to a cold period
- many seeds will only germinate if they are first cold stratified
- Nastic movements

- Sensitive plant (Mimosa pudica)

30. Life history
30.1. Allocation
- Life history characteristics
Control allocation to and timing of reproduction and growth
- Age at first reproductive event
- Reproductive lifespan and age-specific mortality rates
- Number and size of offspring
- Life span
- Annuals (\(\approx 21\%\) of N.A. species)
- Biennials (\(\approx 2\%\) of N.A. species)
- Perennials (\(\approx 77\%\) of N.A. species)
- Variation in life history
- sexual versus asexual reproduction (clones)
- frequency of reproduction:
- iteroparous
- Reproducing more than once
- semelparous
- Reproduce once and die
- Semelparous example, Agave paryi

- Life expectancy
Variations in the life expectancy are related to the probability that an adult survives from one year to the next.
- In unpredictable environments we find more annuals}
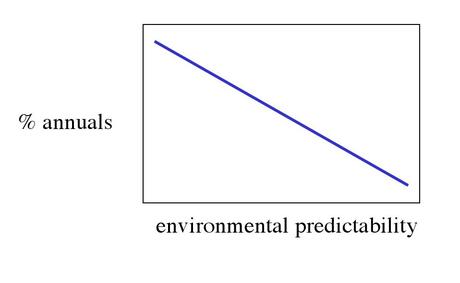
30.2. Seeds and seedbanks
- When should an annual set seed?
Fecundity schedules in annuals often reflect predictability of the end of the growing season
- predictable
- Reproduction delayed until near the end of season (main meristem transformed into reproductive tissue)
- unpredictable
- Reproduction begins as soon as plants have attained some minimal size (often axillary
- Buffering populations against stochasticity
- Seed banks
If environment is constant, seeds should germinate immediately. However environment often is variable in time.
- Long lived persistent individuals
Adults can often survive environmental conditions unsuitable to establishment and can thus buffer population against environmental stochasticity.
- Seed banks
- Seed germination: Predictable vs unpredictable environments
- Light
- Indicates no shading by competitor.
- Soil moisture
- in some environments, rainfall at germination predicts subsequent rainfall.
- Fire
- A good predictor that subsequent environment will be free of established competitors and high in nutrients
- Phenology
- Vegetative phenology
- Reproductive phenology
30.3. Annual vs perennial
- Annual vs perennial?
Cole’s Paradox. Lamont Cole (1954)
- \(p\) = fraction of plants that survive each year to reproduce
- \(F\) = number of seedlings produced by each plant per year
- Then perennial’s fitness \(\lambda_p = p(F_p+1)\)
- Annual’s fitness \(\lambda_a = pF_a\)
- Annual vs perennial continued
Charnov and Schaffer (1973)
Added possibility that survival from seed to adult was different probability.
- \(c\) = Fraction of first year plants (seeds) that survive
- Then perennial’s fitness \(\lambda_p = cF_p + p\)
- Annual’s fitness \(\lambda_a = cF_a\)
- Annuals should be more fit when \(F_a > F_p + \frac{p}{c}\)
- Demography and life history